The latest biomarker biomarker detection technology progress (1)
The concept of biomarkers was first proposed in 1983. It refers to biochemical indicators that can label changes in systems, organs, tissues, cells, and subcellular structures or functions, and has a very wide range of uses. Since biomarkers can be used for disease diagnosis, judging disease staging, or for evaluating the safety and efficacy of new drugs or new therapies in target populations, they play an important role in drug discovery.
In October 2010, the US FDA issued a document discussing the draft guidelines for biomarkers, indicating that biomarkers have become one of the evaluation tools for clinical research of new drugs (Figure 1). The US NIH provides a more practical interpretation of biomarkers: to evaluate and measure the biological processes, pathological processes, and pharmacological responses to therapeutic interventions, including nucleic acids, proteins, and metabolic derivatives. Molecules can be potential biomarkers. Protein is the executor of biological functions, located at the functional end of the central law, providing the most direct basis for basic medicine, translational medicine and precision medicine, and thus the most widely researched and applied marker. Therefore, the detection technology of protein markers is also the most important. So far, antibodies are still the most fundamental basis for the detection of protein biomarkers. Many methods are based on antibodies and are constantly updated and developed. This review intends to systematically analyze and compare existing protein biomarkers from an application perspective. The detection technology, combined with the development of new technologies, looks into the future of biomarkers.

Figure 1. Field of Translational Medicine Research for Biomarker Applications

Figure 2. Application of biomarkers in drug discovery
Protein biomarkers can help people understand biological processes and can be applied to high-risk assessment of disease, early diagnosis, detection and localization, prognosis, treatment response, and recurrence monitoring. In the early diagnosis, in the case of tumor research, biomarkers can provide predictive, diagnostic, and prognostic information. Especially in the recent development of personalized treatment and differential diagnosis techniques, the role of biomarkers is becoming increasingly important. Therefore, biomarkers are the focus of attention for both scientific research and pharmaceutical companies. Biomarkers can provide critical insights into the mechanism of action of drugs, how they affect targets, and downstream effects (Figure 2), as well as the toxicity and side effects of drugs or treatments (such as hepatotoxicity) in early drug studies. Nephrotoxicity, cardiotoxicity, muscle toxicity, etc.) This is very important for drug development. According to statistics, the most important reason for the suspension of drug development process comes from drug safety evaluation or toxicology test failure (Figure 3, about 33 %), and the lack of efficacy is only in the second position (26%).

Figure 3. Analysis of the causes of drug development failure
New therapeutic strategies that have emerged in recent years, such as chimeric antigen receptor T cells (CAR-T) for the treatment of acute lymphoblastic leukemia, have also given new insights into the use of biomarkers. Since CAR-T cells are themselves immunogenic substances, when they are introduced into patients, they often produce very serious side effects, such as Cytokine Releasing Syndrome (CRS), in addition to therapeutic effects. People's understanding of CRS has a very important connection with a star patient called Emily Whitehead. Emily's important position in the history of treatment is that in her previous treatments, there have been consecutive deaths, and she has had a serious inflammatory response. Fortunately, the treatment team used anti-inflammatory drugs to help Emily survive the desperate high fever. Eventually Emily survived. So far, Michel Sadelain (the naming person of CAR-T technology, now working for JUNO) and other pioneers began to apply protein multi-factor detection technology to monitor cytokine biomarkers during treatment, and found the key cytokine CRP after treatment. The level of expression is a good predictor of the risk of CRS (Figure 4, Reference 2). Severe cytokine release syndrome occurs only when the expression level of CRP factor exceeds 20 mg/dl. In the successful CAR-T treatment case from China, protein multi-factor detection technology was also successfully applied (data not shown).
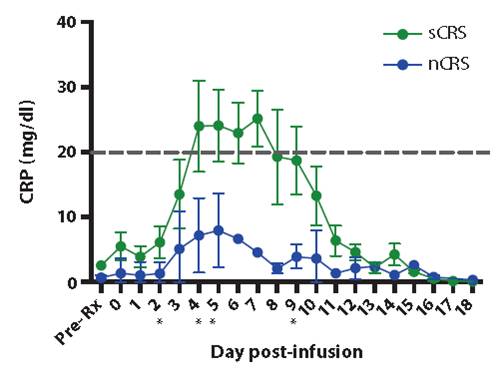
Figure 4. CRP marker monitoring cytokine release syndrome
To download the full text, please click here to visit Baidu Library >>
Next Latest biomarker biomarker detection technology progress (2)
Kn95 5 Layers Mask,Kn95 Filters Face Mask,Kn95 5 Layers Cup Facemask,Kn95 5 Layers Fold Flat Facemask
Jiangmen anjian biotechnology co. LTD , https://www.jmanjianmask.com