This product is not processed by lactic acid fermentation but by artificial formula. The processing technology is as follows: 1. Raw material treatment: use unripe raw papaya, peeled, seeded, sliced, cut the papaya and cut into 0.2-0.3. Centimeter slices are ready for use. 2. Ingredients: Add 2-3% salt, 1% sweetener, 0.8-1% citric acid, 0.05% calcium chloride, 0.05% potassium sorbate, and a small amount of lemon yellow pigment in 50 kg water. After the above additives are dissolved and heated and boiled, the papaya pieces are added. 3. Impregnation: Papaya slices are immersed in the liquid for about one week. Care should be taken to ensure that the feed is immersed in the raw materials. During the impregnation process, the raw materials gradually absorb the various flavors in the feed until the solids and liquids reach equilibrium. After that, the concentration of the feed solution no longer changes and the impregnation finishes. 4. Drying: Remove the melons into the feed solution and send them to the drying room. Require 60-65 degrees Celsius, 2-3 hours of drying time, and the drying time is short. Dry the surface moisture, otherwise the dried papaya strips will not be brittle. Now. Water content control is about 30%. 5. Packing: can be sealed in plastic bags or plastic boxes. Finished product requirements: lemon yellow, sweet and sour food, slightly salty, crisp and delicious.
1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff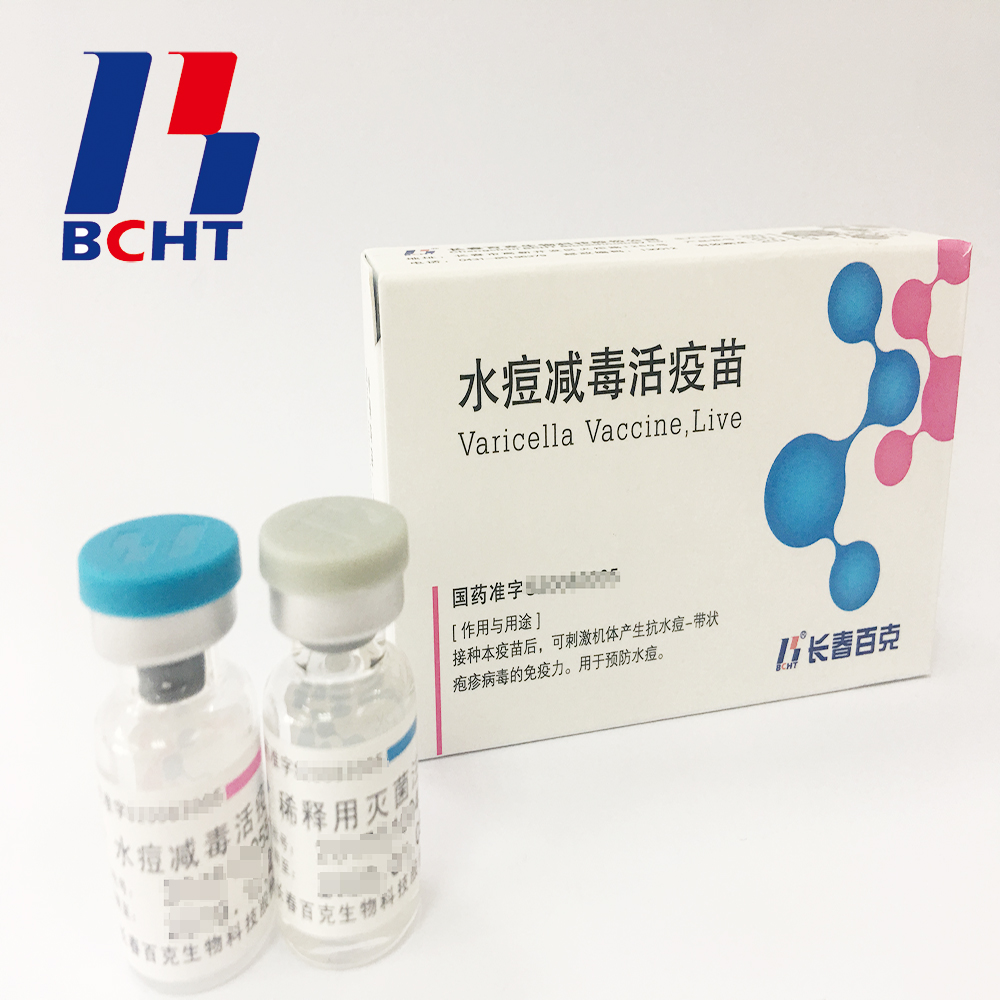 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
Varicella Vaccine(Live)
Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live
Changchun BCHT Biotechnology Co. , http://www.ccbcht.com
 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.