Mesenchymal stem cell expansion using a New Brunswick S41i CO2 constant temperature shaker
The cell culture cycle was 12 days, and daily sample analysis was performed to compare cell growth status, biochemistry, and metabolite indicators. Cell density comparison showed that adipose-derived mesenchymal stem cells were cultured in a logarithmic growth phase (4 days) and a plateau (9 days), and the number of cells expanded under a conical flask was 1.6- higher than that in a rotating flask. 1.8 times. Analysis of daily metabolites showed that the ammonia level in the culture flask was higher in the early culture, but this phenomenon was not observed in the conical flask, which may be caused by stem cell damage caused by the shear force generated by the stirrer.
Finally, by CD44 and CD90 stem cell marker analysis, mesenchymal stem cells have the ability to differentiate into adipocytes or bone cells, confirming the high quality of adipose-derived mesenchymal stem cells expanded using Erlenmeyer flasks.
A Novel Method for the Expansion of Mesenchymal Stem Cells using an Eppendorf New BrunswickTM S41i CO 2 Incubator Shaker
Khandaker Siddiquee and Ma Sha, Eppendorf Inc., Enfeld, CT, USA
Abstract
The expansion of stem cells, including mesenchymal stem cells (MSC), has been successfully demonstrated using microcarrier-based small bioreactors such as spinner flasks. In this study, we explored a simple alternative for microcarrier-based MSC expansion using conventional shake flasks. Method relies on a new type of CO2 incubator with built-in shaking capability, ie New Brunswick S41i CO 2 Incubator Shaker. The expansion of adipose-derived mesenchymal stem cells (AdMSCs) was compared between shake flasks and spinner flasks using microcarriers. The AdMSCs We are seeded at a density of 3x103 cells/cm2 in both setups, each containing 0.5 g plastic microcarriers and 40 ml of stem cell growth medium.
The cell culture comparisons were conducted for 12 days and samples were collected daily for analysis of cell growth, biochemistry and metabolites. Cell density comparisons revealed that AdMSCs cultured under shake flask conditions achieved between 1.6 to 1.8-fold higher cell count over spinner flask culture during Early log phase (day 4) and stationary phase (day 9) of growth. Daily metabolite organizations revealed that the spinner culture had elevated ammonium levels early in the culture not seen in the shake flask culture, indicating possible stem cell damage due to the shear Force generated by the spinner rod.
Lastly, the AdMSCs expanded using the shake flask method remained high quality stem cells, which was evident by CD44 and CD90 stem cell marker assays and their ability to differentiate into either adipocytes or osteocytes.
Introduction
Stem cells are undifferentiated cells which have the capability of self-renewal and the potential to divide for a long period of time. They have the ability to differentiate into various specialized cells when appropriate growth factors and conditions are provided. Stem cells can be broadly classifed As: embryonic, adult, and induced pluripotent stem cells (iPS). adult stem cells can be further characterized by their tissue of origin, such as: hematopoietic, mammary, intestinal, mesenchymal, endothelial, neural, and hair follicle stem cells. Of the studies performed on adult stem cells synthetic either hematopoietic or adipose-derived mesenchymal stem cells1. Like other adult stem cells, adipose-derived mesenchymal stem cells (AdMSCs) express all of the common stem cell markers and can be differentiated into various types of Specialized cells under appropriate growth conditions. AdMSCs have advantages over other mesenchymal stem cells (MSCs), since they can be isolated in large q Uantities from fat tissue and are resistant to apoptosis2.
MSCs have enormous advantages for regenerative medicine, drug screening and drug discovery, their applications are limited by the quantities required for industrial or clinical applications3. In this study, we developed a simple shake flask technique to expand MSCs on microcarrier beads which can be Used to scale-up into large-scale bioreactors. The microcarrier shake flask culture, which requires both agitation and CO2 gas control, was conducted in the Eppendorf New Brunswick S41i CO2 incubator shaker.

The New Brunswick S41i CO 2 Incubator Shaker, designed for both non-adherent and adherent cell culture applications, combines the precise temperature and CO2 control of an incubator with the reliable New Brunswick laboratory shaker drive mechanism. Key features include sealed inner/outer doors, High-temperature disinfection, and reduced CO 2 consumption compared to competitor models4.
Materials and Methods
Initial cell culture in T-Flasks
AdMSCs were obtained from ATCC ® (PCS-500-011) at passage 2 and cells were seeded at a density of 5,000 cells/cm2 into a T-75 cm2 fl ask (Eppendorf) using 15 ml of mesenchymal stem cell basal medium (ATCC Supplemented with 2% fetal bovine serum, 5ng/ml rh FGF basic, 5ng/ml rh acidic, 5 ng/ml rh EGF and 2.4 mM L-Alanyl-L- Glutamine (ATCC). Cultivation of cells on microcarriers Prior to start Of the experiment, 0.5 g of 125-212 micron polystyrene microcarriers (SoloHill ® ) (180 cm2 for a 50 mL culture) was transferred into a siliconized (Sigmacoat ® ; Sigma) 250 ml spinner fl ask (Corning ® ) and shake fl asks (Schott®, Duran ® ) along with 25-30 ml of PBS. The fl asks were then autoclaved at 121 °C for at least 30 minutes. Microcarriers were allowed to settle to the bottom of the shake/spinner fl asks and the autoclaved PBS buff ers were carefully aspirated with the electronic pipetting aid easypet ® (Eppendorf) equipped with a 25 or 50 mL pipette. The AdMSCs were initia Lly seeded at a density of 3x103 cells/cm2 into both fl asks, each containing 40 ml of basal mesenchymal stem cell medium. For the initial attachment of cells, the agitation speed of the New Brunswick S41i CO 2 incubator shaker and rotation speed of the Spinner (housed inside of an Eppendorf Galaxy® 170 R CO 2 incubator) were both kept at 50 rpm and incubated for 2 hrs at 37 °C with 5% CO 2 . After incubation, the cell culture volume was adjusted to 50 ml total with 10 ml of medium containing serum to reach af nal FBS concentration of 4% and targeted f nal concentration of growth supplements (10 ng/ml f nal concentration of rh FGF basic, rh FGF acidic & rh EGF and 4.8 mM f nal concentration of L -Alanyl-LGlutamine). Following the addition of FBS and growth supplements, the rotation speed of the spinner and the agitation speed of New Brunswick S41i CO 2 incubator shaker were both raised to 70 rpm. After 18 to 24 hrs of incubation, 1 ml Of homogeneous samples containing both media and Microcarriers were collected for microscopic observations, cell counting as well as biochemistry analysis. Cell counting
The actric acid solution containing crystal violet (0.1% crystal violet in 0.1 M citric acid solution) equal to the volume of supernatant removed from the tube. The contents of microcarrier beads were counted by hemocytometer. The tube were incubated for 1 hr or overnight at 37 °C and vortexed for a few seconds to release the stained nuclei. The nuclei were counted with hemocytometer.
Biochemistry and metabolites analysis The supernatants collected during cell counting were used for biochemistry and metabolite measurements using an automated YSI ® 2950 Bio-analyzer.
Stem cell surface marker assay
To assess the quality of AdMSCs after expansion and to conf rm that the stem cell markers were retained during the microcarrier-based culture, CD44 and CD90-specif c fl uorescent immunoassays were performed using the following procedure. 5 ml samples were collected from both the Spinner and shake fl asks near the end of microcarrier culture. After the microcarriers settled to the bottom, the supernatants were removed and the microcarrier beads containing cells were gently washed 3 times with PBS at room temperature. Cells on the microcarrier beads were then f xed With 4% paraformaldehyde for 30 minutes followed again by PBS washing 3 times. Cellcontaining microcarrier beads were blocked with 5% FBS at room temperature for 1hr and immunostained with FITC-conjugated antihuman CD44 (BioLegend ® ) and APC-conjugated antihuman CD90 (BioLegend ® Antibody solutions, also for 1hr at room temperature. The beads were washed 5 times with room temperature PBS for 5 min and visualized using a n EVOS ® FL fl uorescence microscope.
Stem cell differentiation assays
AdMSCs were harvested from both shake and spinner flasks into 50 ml tubes. Once the microcarrier beads settled to the bottom of the tube, the supernatants were removed and cells were washed with DPBS. Afterwards, the microcarrier beads were treated with 5 ml of prewarmed trypsin - EDTA solution at 37 ° C for 10 min. during incubation, the tubes were occasionally vortexed for 2 sec and then neutralized by adding an equal volume of trypsin neutralizing solution. Microcarrier beads were a llowed to settle to the bottom of the tube and the Microcarrier beads were washed 2-3 times with DPBS and as much supernatant as possible was collected into a 50 ml tube. Following washing, AdMSCs were collected to bottom of the tube by centrifugation at 120 xg for 5 Min and resuspended in 5 ml of mesenchymal stem cell medium. Cells were seed at a density of 18,000 cells/cm2 into a 24-well plate. Adipocytes and osteocyte differentiations were performed on Those cells using differentiation assay kits from ATCC. Adipocyte and osteocyte differentiated cells were identifed by cell-type specifc staining with either Oil red O or Alizarin red S kits (ScienCell ® ) according to manufacturer instructions and visualized using an OLYMPUS ® CK40 microscope.
Results and Discussion
To compare between shake flask and spinner flask cultures, AdMSCs were seeded at a density of 3x103 cells/cm2 in both systems. Cell culture comparisons were conducted for 12 days and samples were collected for cell growth, biochemistry and metabolite analysis daily. Cell growth comparisons Revised that AdMSCs cultured under shake flask conditions achieved between 1.6 and 1.8-fold higher cell count over spinner flask cultures during early log phase (day 4) and stationary phase (day 9) of growth (Figure 1A). Biochemistry and metabolite analysis revealed that Glucose concentrations decreased from 1.09 g/l to 0.548 g/l (for shake flask culture) and 0.798 g/l (for spinner culture), when lactate concentrations increased from 0.042 g/l to 0.396 g/l (for shake flask culture) And 0.259g/l (for spinner culture) after 12 days of culture (Figure 1B & C). The higher glucose consumption and lactate production rate seen in the shake flask culture supports the fnding that the stem cells grew At a faster rate under the shake flask conditions. further, during early growth phase (day 4); the amount of ammonium accumulated in spinner flask culture (2.4 mM) was 1.8-fold higher than shake flask culture (1.3 mM) (Figure 1D The smear of the culture process, which indicates the slower growth by the spinner method could be a result The fact that spinner culture had elevated ammonium levels early in the culture not seen in the shake flask also indicates possible stem cell damage due to shear force by the spinner rod. The spinner rod was observed to display a "stop & go" motion at low speeds; precise speed control is not possible, especially at low rotation speeds.
To determine whether or not AdMSCs retained their stem cell properties during their growth under shake flask conditions, immunostaining of stem cell surface markers and differentiation assays were performed. Microcarrier beads that contained AdMSCs were immunostained with stem cell surface marker antibodies such as: FITC conjugated antihuman CD44 and APC-conjugated antihuman CD90 and revealed that AdMSCs retained stem cell surface markers during growth under shake flask culture condition (Figure 2A&B). For the adipocyte and osteocyte differentiation assays, AdMSCs were collected from the microcarrier beads and seeded into 24 well plates that After 17 days of culture, the plates were stained with Oil Red O or Alizarin Red S staining solutions, respectively. Microscopic observation indicated that most of the AdMSCs from shake flask culture differentiated into either adipocytes or osteocytes successfully (Figure 3A&B).
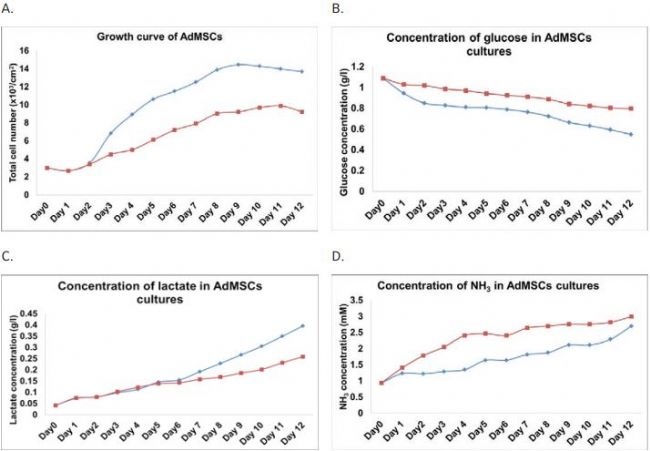
Figure 1. Comparison of AdMSCs between shake fl ask and spinner fl ask cultures: A) growth; B) glucose utilization; C) lactate production and D) ammonium production. (ïµ) shake fl ask and (ï®) spinner fl ask.
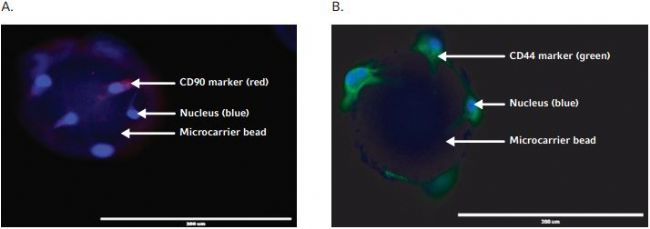
Figure 2. Stem cell marker identif cation assay for AdMSCs expanded on microcarriers in shake fl ask. A) AdMSCs on microcarrier beads are positive for CD90 stem cell marker, as indicated in red by fl uorescence imaging. B) AdMSCs on microcarrier beads are positive For CD 44 stem cell marker, as indicated in green by Fluorescence Imaging. Blue color indicates stem cell nuclear staining by DAPI.
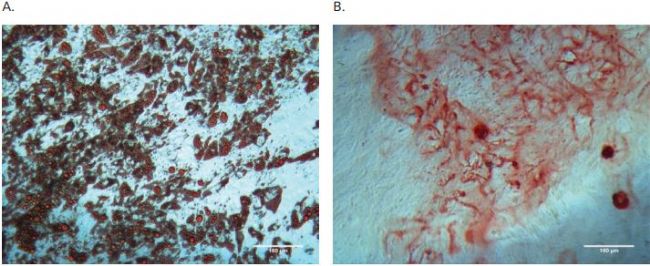
Figure 3. Diff erentiation assays for AdMSCs expanded on microcarriers in shake fl ask. A) Adipogenic diff erentiation formed lipid droplets as indicated by Oil red O positive staining. B) Osteogenic diff erentiation caused calcium mineralization of extracellular matrix as indicated by Alizarin Red S Positive staining.
Conclusions
The novel method relies on a new type of CO2 incubator with built-in shaking capability, such as the New Brunswick S41i CO 2 Incubator Shaker. The New Brunswick S41i reduces shearing, eliminates potential cell damage by the spinner rod, decrease the risk of contamination associated with inserting a magnetic stirrer base into the CO 2 incubator and reduces experimental complexity. This method also greatly increases the cell culture capacity The number of shakes can be placed in the New Brunswick S41i simultaneous. In the case of spinner flask culture, a typical incubator without active cooling can only handle the heat emitted from a very limited number of magnetic stirrer bases before causing temperature setpoint overshoot, a Signifcant limitation to the scale-up potential of the spinner method. This reinforces the superiority of New Brunsw I s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s s
References
1. Zhang et al. "Mechanisms Underlying the Osteo- and Adipo-Differentiation of Human Mesenchymal Stem Cells". The Scientifc World Journal (2012). Volume 2012; Article ID 793823;
2. Nastran el al. "Osteogenic Differentiation and Osteochondral Tissue Engineering Using Human Adipose-Derived Stem Cells." Biotechnology progress (2013) Volume 29. No. 1: 176-185;
3. Schop et al. “Expansion of human mesenchymal stromal cells on microcarriers: growth and metabolism†J. Tissue Eng Regen Med (2010); Volume
4. No. 4; 131-140. 4. Kohlstrom et al. “Hybridoma and CHO Cell Culture using New Brunswick S41i – An Environmental Friendly, Low Emission Incubator Shakerâ€. Eppendorf Application Note AA257, Nov. 2012.
5. Schop D. "Growth and Metabolism of Mesenchymal Stem Cells Cultivated on Microcarriers", Ph.D. Thesis 2010, University of Twente, The Netherlands.
Dental sealing machine,sealing machin dental
Foshan Ja Suo Medical Device Co., LTD , https://www.fjoralinstrument.com