FDA recognizes innovative gene therapy for liver disease as a breakthrough therapy
Alnylam Pharmaceuticals, a leading RNAi treatment company, recently announced that its research and development givosiran has received FDA-issued breakthrough therapy approval. Givosiran is an RNAi therapy against aminolevulinic acid synthase 1 (ALAS1) for the prevention of acute hepatic porphyria (AHP).
Acute Hepatic Porphyrias (AHP) is a rare metabolic disorder, mainly autosomal dominant, caused by a genetic mutation in one of the eight enzymes responsible for heme synthesis. Acute hepatic porphyria includes several subtypes: acute intermittent porphyria (AIP), hereditary porphyria (HCP), and variegate porphyria (VP). AHP patients who are exposed to certain drugs, foods, or hormonal changes strongly induce an enzyme in the heme biosynthetic pathway, aminolevulinate synthase 1 (ALAS1), which results in a neurotoxic heme intermediate. Accumulate, causing the onset of the disease.
AHP patients develop a range of life-threatening symptoms, such as severe abdominal pain, peripheral and autonomic neuropathy, neuropsychiatric symptoms, and skin damage that can lead to paralysis and death if left untreated or treated. Therapeutic methods for preventing the onset of AHP have not yet been approved, and the only approved acute exacerbation therapy is hemoglobin for injection (Panhematin or Normosang), a heme preparation derived from human blood. Heme needs to be treated by large or central vein injection and is associated with many complications, including thrombophlebitis or coagulopathy.
Givosiran is an RNA interference (RNAi) therapy targeting ALAS1. It has been approved by the European Medicines Agency (EMA) PRIME, as well as the orphan drug in the European Union and the United States. It is used to treat acute hepatic porphyria and has the potential to become A new treatment for preventing recurrence. RNAi is a new approach to drug discovery and development that uses small interfering RNA (siRNA) molecules to target specific mRNAs and silence them to prevent the production of pathogenic proteins. RNAi therapy has the potential to cure diseases radically. Its findings are considered to be “a major scientific breakthrough that occurs only once every decade†and are one of the frontiers of rapid development in biology and drug discovery today, and were awarded the 2006 Nobel Prize in Physiology or Medicine.
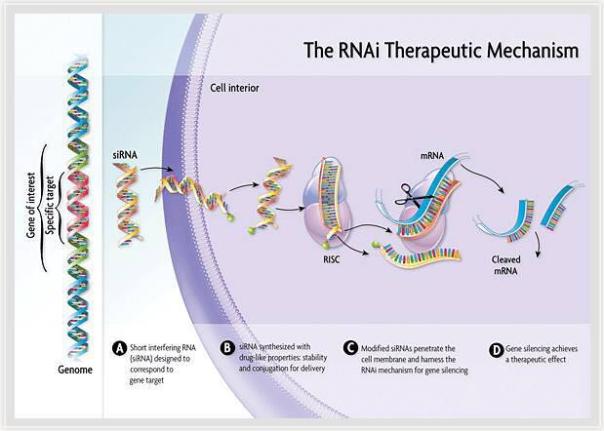
â–² RNAi mechanism of action (Source: Alnylam official website)
Transparent Dressing MDK-TD-01
China Transparent Dressing,Transparent Membrane Dressing supplier & manufacturer, offer low price, high quality Transparent Medical Dressing,Clear Surgical Dressing, etc.
Transparent Dressing,Transparent Membrane Dressing,Transparent Medical Dressing,Clear Surgical Dressing
Henan Maidingkang Medical Technology Co.,Ltd , https://www.mdkmedicales.com