Amicus Pompeii's new drug demonstrates long-term positive effects
Amicus Pompeii's new drug demonstrates long-term positive effects
February 09, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, WuXi PharmaTech partner Amicus Therapeutics announced the latest results of a Pompe disease clinical trial at a scientific conference in San Diego. Studies have shown that Amicus' innovative treatments significantly improve patient walking performance and stabilize patient lung function during a year of follow-up.

Pompe disease is a rare genetic disease. According to the United Pompe Foundation, approximately 5,000 to 10,000 people worldwide are affected by the disease. These patients lack an enzyme called acid alpha-glucosidase (GAA), which causes abnormal accumulation of glycogen in the lysosomes of muscles and other tissues, making muscles weaken over time. Therefore, the patient's daily walking ability will be affected, and the heart and lung function will be affected. Currently, patients with Pompe disease rely mainly on enzyme replacement therapy (ERT) for disease control.
A combination therapy brought by Amicus is expected to improve the condition of these patients. The therapy consists of ABT200 and AT2221, a recombinant human acidic GAA (rhGAA) with optimized carbohydrate structure (especially mannose-6-phosphate) that enhances muscle uptake by the patient; the latter is a A molecular chaperone that acts as a stable enzyme replacement therapy in the blood to help the enzyme remain active. The two are co-administered and are expected to have a good synergistic effect.
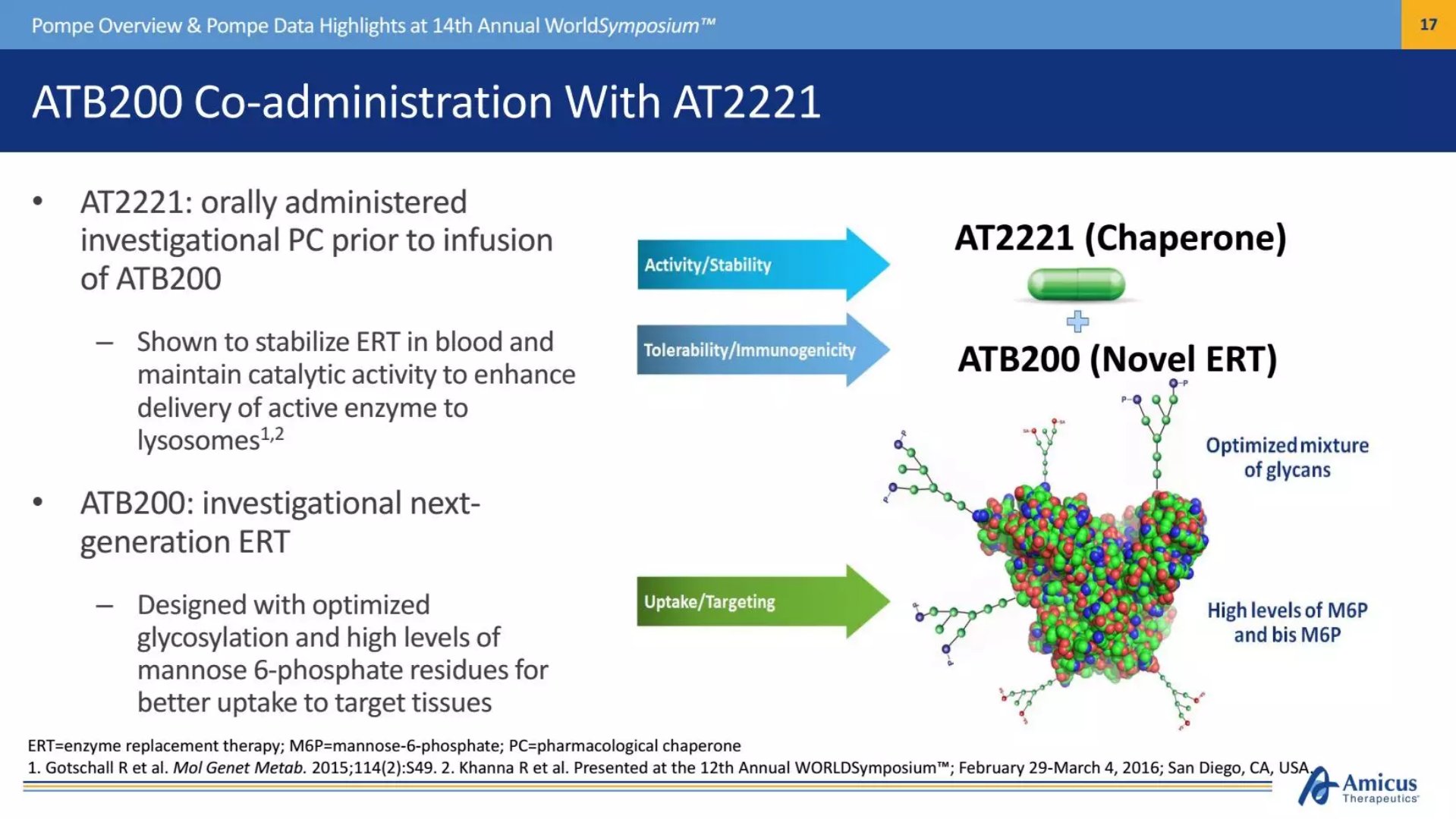
â–²Two drugs for this combination therapy (Source: Amicus official website)
In a clinical trial called ATB200-02, the researchers evaluated the efficacy of the combination therapy in 20 patients. In October last year, the study published preliminary positive results indicating improved walking and lung function. The data released this week further validates the effectiveness of the therapy.
Studies have shown that as treatment time increases, the patient's walking test scores at 6 months, 9 months, and 12 months steadily increase. In terms of lung function, with reference to indicators such as forced vital capacity (FVC), the patient's key values ​​showed signs of stabilization or improvement after 12 months of treatment.
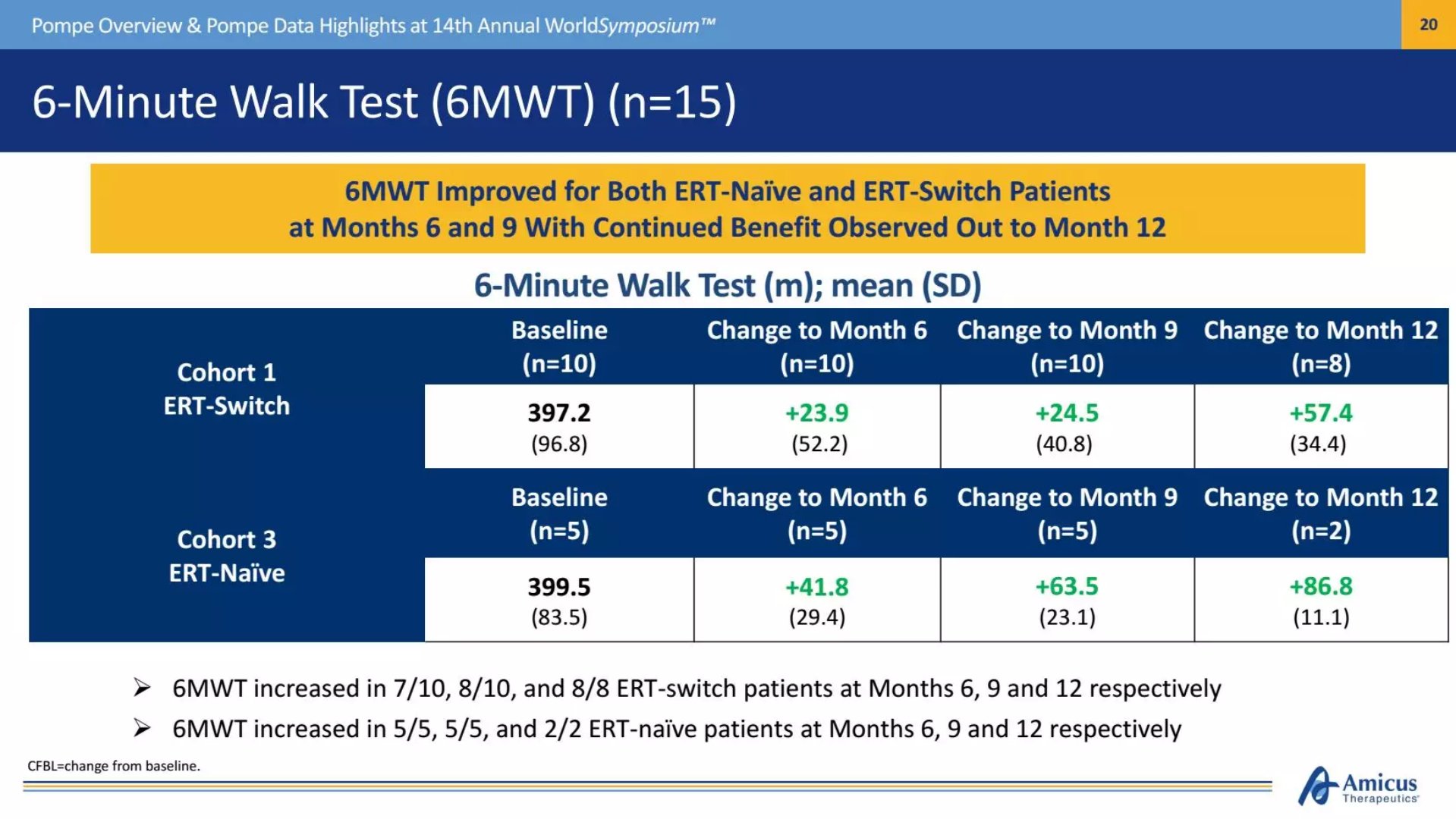
â–²The patient's walking ability is steadily increasing (Source: Amicus official website)
Readers familiar with Amicus Therapeutics may know that one of the sons and daughters of Dr. John Crowley, Chairman and CEO, is also a Pompeii patient, which is one of the reasons why his founding company developed new drugs. We congratulate Amicus on its positive progress in clinical trials and look forward to its success in bringing benefits to rare patients!

Reference materials:
[1] Amicus Pompe Overview & Pompe Data Highlights at 14th Annual WorldSymposiumTM
[2] Amicus official website
Medical Surgical Instruments,Electrode Suction Irrigation,Gun Suction Tube,Stainless Steel Laparoscopic
ZHEJIANG SHENDASIAO MEDICAL INSTRUMENT CO.,LTD. , https://www.sdsmedtools.com