Long-term removal of wrinkles, the new drug phase 3 clinical arrival to the primary end
Long-term removal of wrinkles, the new drug phase 3 clinical arrival to the primary end
December 06, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Revance Therapeutics today announced the positive top-line results of its next-generation neuromodulator, Daxibotulinumtoxin A (RT002), in two key phase 3 clinical trials, which showed that the new drug significantly relieved the severity of moderate to severe eyebrow lines. . It showed good safety and tolerability in both studies.

The eyebrow is between the eyebrows and the nose. The eyebrow pattern is often referred to as the "flaw line". This vertical line formed between the eyebrows is a single line, two or more lines. When frowning, the muscles in the lower part of the forehead contract downwards, causing the skin between the eyebrows to shrink. These lines are formed by repeated effects due to lack of elasticity of the skin. Age, sun exposure and genetic factors are also part of the influencing factors. RT002 blocks nerve impulses and temporarily suppresses muscle movements that cause wrinkles, making the skin smoother and more refreshing.

▲This new drug is expected to alleviate the severity of “Frown Eyebrow†(Source: Pixabay)
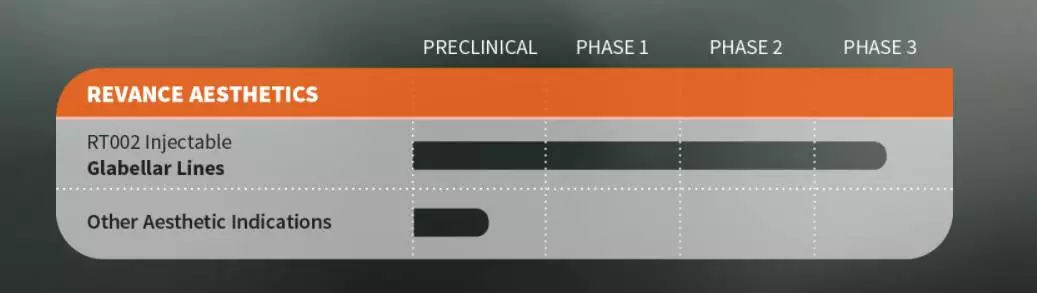
RT002 is a next-generation neuromodulator used in the treatment and treatment of symptoms including eyebrow lines, cervical dystonia and plantar fasciitis. Revance's proprietary peptide technology makes it possible for RT002 to be the first long-acting neuromodulator to be effective for six months.
The SAKURA clinical program includes two randomized, double-blind, placebo-controlled, key trials of SAKURA 1 and SAKURA 2, both of which have the same design, evaluating single-use RT002 for moderate to severe eyebrows in adults aged 18 to 75 years. The safety and effectiveness of the pattern. The SAKURA 1 and SAKURA 2 trials enrolled 609 patients in 30 locations in the United States and Canada. In both trials, patients were randomized to receive either RT002 (40 U) or placebo in a 2:1 ratio.
Both the SAKURA 1 and SAKURA 2 studies reached the primary composite end point, and RT002 statistically significantly reduced the severity of the eyebrow lines compared with placebo. At week 4, treatment with RT002 achieved wrinkle-free or mild wrinkles, and the proportion of patients who scored 2 points higher than baseline was 73.6% (placebo 0%) and 74.0% in SAKURA 1 and SAKURA 2, respectively (consolation) Agent 1%) (p <0.0001). At the same time, 88% (SAKURA 1) and 91% (SAKURA 2) of patients receiving RT002 expressed satisfaction or were very satisfied with the treatment experience.
â–²In terms of beauty products, RT002 is Revance's leading research drug (Source: Revance official website)
RT002 treatment also reached a critical secondary clinical endpoint and was highly statistically significant at each time point during the 24-week assessment period. At an additional critical secondary endpoint, the median time to restore baseline wrinkle severity to baseline for RT002 patients assessed by the physician and patient was approximately 27 weeks (SAKURA 1:27.7 weeks, SAKURA 2:26.0 weeks).
“We are very pleased with the positive top-line results of these SAKURA results, which reinforce the findings of the BELMONT Phase 2 active comparative study. These results suggest that we can create a long-acting neuromodulator with a duration of six months compared to Under the current available products are three to four months," said Dan Browne, co-founder, President and CEO of Revance Therapeutics. "We look forward to providing patients, healthcare professionals with new, next-generation, long-acting neuromodulators. Treat eyebrow lines."

â–² Mr. Dan Browne, co-founder, President and CEO of Revance Therapeutics (Source: Revance Official Website)
“SAKURA 1 and SAKURA 2 both show that RT002 provides consistent long-term performance, which is unprecedented in the neuromodulators we have seen in the past 30 years,†said SAKURA Principal Investigator, Clinical Professor at the University of British Columbia. Dr. Jean D. Carruthers said: "These data confirm the enhanced effect of this novel neuromodulator in prolonging efficacy and patient response, requiring only two treatments per year, and RT002 may alter the current state of neuromodulation therapy.
In addition to SAKURA 1 and SAKURA 2, the long-term safety test SAKURA 3 is fully registered and is expected to be completed in the second half of 2018. If SAKURA 3 is successfully completed, the company plans to submit a biologics license application in the first half of 2019. After FDA approval, RT002 will be launched in the United States in 2020.
Reference materials:
[1] UPDATED: Watch out Allergan: Revance's wrinkle-reducer beats Botox in PhIII trials
[2] Revance's RT002 Meets Primary and All Secondary Endpoints, Achieves 6-Month Duration in Pivotal SAKURA Phase 3 Trials for Glabellar Lines
Low Residue Void Open Security Labels,Void Security Labels,Void Anti-tampering Labels,Low residue security labels
Wenzhou Haoshi Light Industrial Products Co., LTD , https://www.economicseals.com