Why do stray cancer cells like liver?
As the saying goes, "Heart and liver baby", the importance of the liver to the human body is self-evident. And such an important liver is often the target of tumor metastasis.
Why is the liver always injured? There are naturally human anatomical reasons, but the liver's own tissue properties are absolutely difficult to blame.
Recently, Jae Lee and Gregory Beatty of the University of Pennsylvania found that pancreatic cancer cells in early stage will stress the secretion of IL-6 from surrounding tissues, transform the liver microenvironment, increase the number of myeloid cells, and cause extracellular matrix deposition. Cancer cell metastasis. Related research was published in Nature [1].

Tumor metastasis, especially distant metastasis such as liver metastasis, has been an important cause of cancer death [2]. Different types of tumors also have different metastasis patterns. Like breast cancer, they often metastasize to bone, lung, liver and brain, while lung adenocarcinoma prefers brain, bone, adrenal gland and liver.
In various metastases, the liver and lungs can be said to be the two organs most often lying. They are both a venous blood and lymph that receives the whole body through the right heart, and a blood that receives the digestive tract through the portal vein. It is the first capillary network that the tumor cells entering the blood circulation pass through. This also gave birth to the hemodynamic hypothesis of tumor metastasis [3].
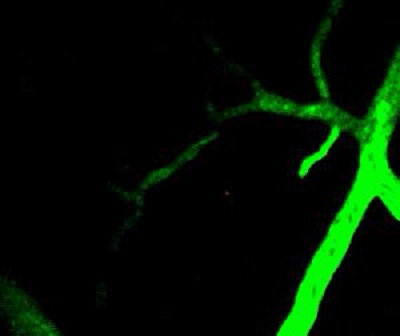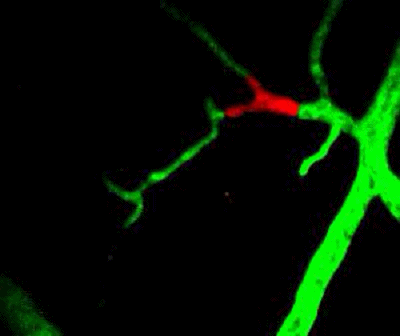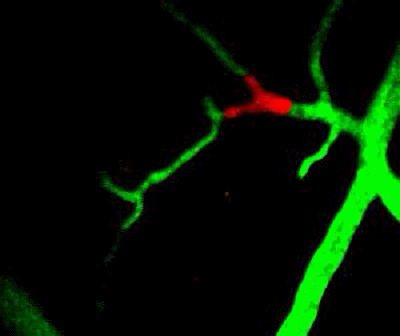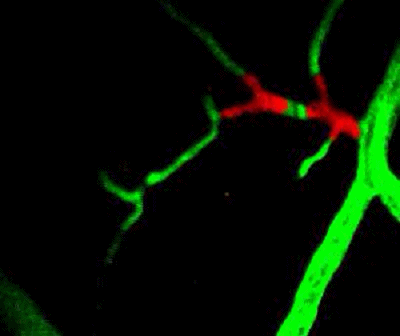
Tumor cells (red) are stuck in the capillary network
However, the hemodynamic hypothesis does not solve all the problems. For example, Stephen Paget found that 32.8% of patients had liver metastases, but only 2.3% of patients had spleen metastasis in autopsy of 735 breast cancer patients [4]. The hemodynamic hypothesis does not explain why spleen metastasis is so small. To this end, Paget proposed the seed-soil hypothesis.
The seed-soil hypothesis compares metastatic cancer cells to seeds, and compares various cells and matrices in target organs to soil. Only suitable seeds encounter suitable soils to root and germinate, and grow into tumor metastases [5] .

Among various tumors, pancreatic cancer prefers the liver, 85% of pancreatic cancers metastasize to the liver, and 74% only metastasize to the liver [6]. Why is the liver so favored by pancreatic cancer? Jae Lee and Gregory Beatty et al.
The researchers used KRAS and TRP53-mutant spontaneous pancreatic cancer mice to compare mice that had developed pancreatic cancer over 16 weeks of age, and liver tissues of mice at 8 to 10 weeks of age and in precancerous lesions. It was found that the number of myeloid cells in the liver of mice that had grown into pancreatic cancer increased, and the expression deposition of extracellular matrix fibronectin and type I collagen increased.
Increased myeloid cells and deposition of extracellular matrix may be preparations for pancreatic cancer for metastasis [7]. Next, the researchers injected labeled pancreatic cancer cells into the two groups of mice. In the livers of mice with pancreatic cancer, the tumor burden of fluorescence is three times that of the control group, which is more numerous, larger, and faster.

The mice with pancreatic cancer have a new tumor burden
By sequencing the hepatocyte mRNA, the researchers identified 275 proteins with differential expression in the two groups of mice, the most significant of which were IL-6–JAK–STAT3-related proteins. Further analysis of STAT3 activation showed that STAT3 in 80-90% of hepatocytes was activated by phosphorylation in mice with pancreatic cancer, compared with 2% in the control group.
What exactly activated these STAT3s? The researchers turned their attention to the IL-6 upstream. The researchers found that in mice that knocked out IL-6, the primary tumor of the pancreas was unaffected, but in the liver, activation of STAT3 was diminished, and expression of myeloid chemokine serum amyloid (SAA) decreased. The accumulation of myeloid cells and extracellular matrices has also decreased, and more importantly, the liver's ability to accept metastatic tumors has decreased. The receptor blocking IL-6 also produced a similar effect.
Further studies have found that these IL-6s that engineer the liver microenvironment are not produced by pancreatic cancer cells, and certainly not by hepatocytes, but by pancreatic cancer cells that are stressed by surrounding normal host cells.

Normal cells secreting IL-6 around pancreatic cancer
To further determine the role of STAT3 in hepatocytes in promoting liver metastasis, the researchers specifically knocked out STAT3 in mouse hepatocytes. Knocking out STAT3 also did not affect the primary tumor of the pancreas, but reduced the ability of the liver to undergo tumor metastasis and prevented the production of liver SAA. The knockout of SAA also reduces metastases in the liver without affecting the primary tumor.

Higher blood SAA levels are associated with poor prognosis
In human pancreatic cancer patients, the researchers found that the level of SAA in the blood increased as well as in mice, and that high levels of SAA were associated with poor prognosis. In five of the seven patients with liver metastases from pancreatic cancer, the researchers did find overexpression of SAA and STAT3 in hepatocytes.
In addition, in patients with non-small cell lung cancer liver metastases and colon cancer patients, the researchers also found an increase in circulating SAA levels and SAA overexpression in hepatocytes. Perhaps pancreatic cancer, a mechanism that modifies the liver microenvironment and promotes metastasis, is also present in other tumors.
Perhaps in the future, we can prevent tumor liver metastasis from being the leading cause of cancer death by targeting the IL-6–JAK–STAT3 pathway in hepatocytes.
Source: Singularity Network
Medical Stockinette,Impervious Stockinette,Surgical Stockinette,Disposable Impervious Stockinette
ShaoXing SurgeCare Medical Products Co., Ltd. , https://www.sxsurgecaremedical.com